Montana ranchers produce some of highest quality beef you’ll find anywhere. But here of late, plant-based proteins and lab grown meat have grabbed the headlines and cattlemen and cattlewomen want to protect the integrity of their industry thru proper labeling of these alternative proteins.
For ranchers like Maggie Nutter, a board member of the U.S. Cattlemen’s Association from Sweetgrass, MT, meat is meat, and beef is bovine.
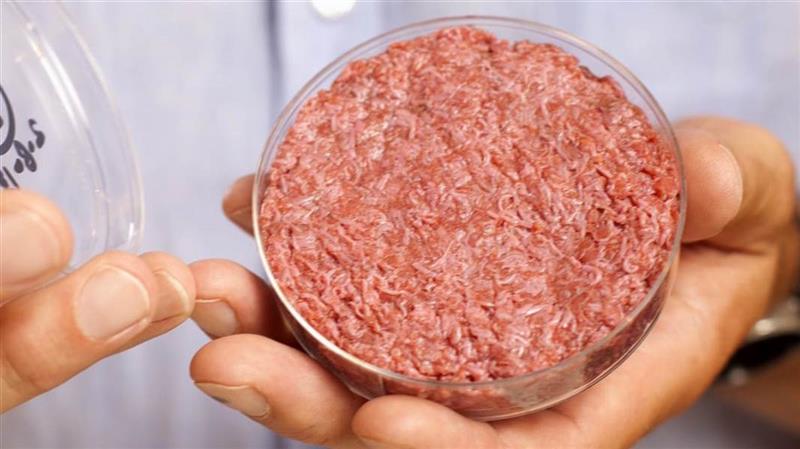
“The U.S. Cattlemen’s policy is that meat is defined as coming from a live animal and harvested in the traditional manner” said Nutter. “It’s not anything that comes from insects, crickets, cultured cells or plants.”
The National Cattlemen’s Beef Association’s president-elect Jennifer Houston says this is why there’s a need to regulate alternative protein.
“We feel very strongly that if the product is going to be called meat especially lab created meat that it needs to be held to the same safety and sanitary standards and nutritional standards that ours are and it’s labeled correctly” said Houston.
That’s why she says NCBA believes the USDA versus the FDA is better suited to regulate these new alternative proteins.
“So, we think that’s the reason that FSIS under USDA should have jurisdiction over that and that’s the comments we’re going to make rather than FDA who’s not had a really good track record of enforcing identity standards over the last 30 years” said Houston.
Nutter says when other products use the term meat or beef they’re taking advantage of the years of hard work ranchers like her and programs like the Beef Check-Off program have put in building beef’s good reputation.
“What it will do is affect our bottom line” said Nutter. “When you start to think about another product coming in and taking a part of your business it’s serious business. Especially, when they clearly state that they’re goal is to eliminate animal agriculture. We have to address it.”
After months of debate among livestock groups and the emerging cell-cultured protein industry, the USDA and FDA have announced an agreement on a joint regulatory framework in which FDA will oversee cell collection, cell banks, cell growth and differentiation.
A transition from FDA to USDA oversight will occur during the cell harvest stage. USDA will then oversee the production and labeling of food products derived from cells of livestock and poultry.
Memphis Meats, a California company aiming to grow cell cultured meat, commended USDA and FDA for the approach saying the regulatory framework plays to the respective strengths of both agencies, while continuing to foster innovation and assure a safe and reliable food system.
The announcement comes after a joint public meeting held last month discussing the use of livestock and poultry cell lines to develop cell-cultured food products. At this meeting, stakeholders shared perspectives on the regulation needed to both foster these innovative food products and maintain the highest standards of public health.
A public comment period has been extended and will remain open through December 26, 2018.
Source: MTN & Northern Ag Network